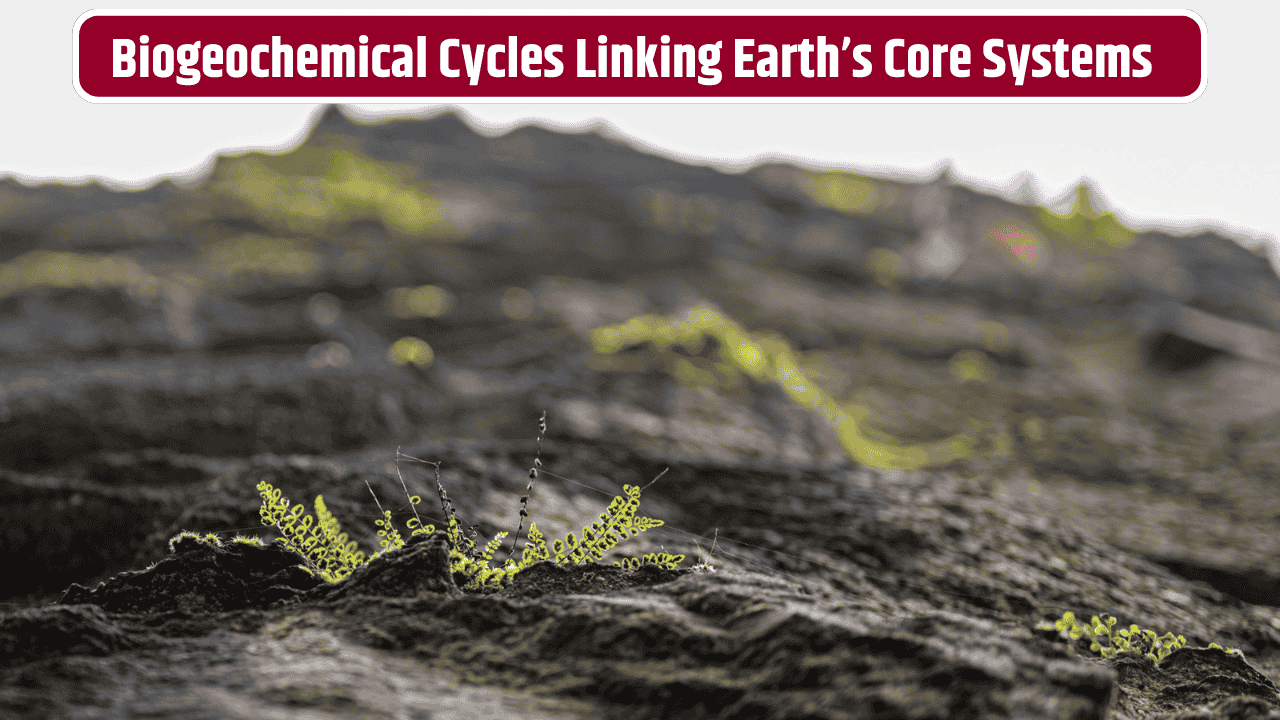
If you’ve ever stood barefoot on damp soil after rain and felt that earthy smell rising up, you’ve already been in the middle of a massive, invisible exchange. Rocks dissolving, soils breathing, waters carrying life and death in equal measure—all of this is tied to what scientists call biogeochemical interactions. Sounds like a mouthful, right? But at its core, it’s just about how rocks, dirt, water, and living things swap elements back and forth, shaping the world we walk on.
Table of Contents
What Are Biogeochemical Interactions?
The word itself breaks down pretty neatly: bio (life), geo (earth), chemical (elements and compounds). Put together, it’s about the flow of elements like carbon, nitrogen, phosphorus, and even metals across living organisms, soils, rocks, and waters. These interactions keep Earth’s systems balanced—without them, soils wouldn’t grow crops, rivers would turn toxic, and the atmosphere would be unbreathable.
Think of it like a barter system: rocks release minerals into water, microbes tweak those minerals, plants slurp them up, and eventually, when those plants (or animals) die, the elements cycle back to the soil and rock.
Rock–Water–Soil Connections
Rocks are the long-term storage lockers of the Earth. When rainwater seeps through cracks, it weathers rocks, pulling out calcium, magnesium, potassium, and iron. Those minerals don’t just vanish—they move downstream into soils and rivers.
Soils, in turn, act like middle managers. They trap nutrients, filter pollutants, and release only what plants or microbes can use. For example, phosphate locked in rock gets freed during weathering, then cycles through soil microbes before reaching plants.
Water is the courier. It doesn’t just move elements around; it transforms them. Oxygen levels, acidity, and temperature in water all influence whether a metal stays dissolved, turns into a solid, or even becomes toxic.
| Element | Rock Source | Soil Role | Water Pathway | Biological Impact |
|---|---|---|---|---|
| Phosphorus | Apatite minerals | Microbial processing | Moves via rivers to oceans | Essential for DNA, ATP |
| Carbon | Carbonates | Organic matter storage | Dissolves as CO2 or bicarbonate | Basis of life, climate regulation |
| Nitrogen | Atmospheric (fixed via bacteria) | Stored in humus | Leaches into groundwater | Protein and chlorophyll formation |
| Iron | Basalts, granites | Bound to oxides | Carried in rivers, redox-sensitive | Helps in oxygen transport (hemoglobin) |
The Role of Microbes
Microbes are the real puppet masters here. In soils, bacteria and fungi decompose organic matter, releasing nitrogen, phosphorus, and sulfur back into circulation. In water systems, microbes alter redox conditions—deciding whether iron stays dissolved or precipitates as rust-colored sludge.
For instance, in wetlands, microbes can strip oxygen from nitrates in a process called denitrification, releasing nitrogen gas back into the atmosphere. That’s why wetlands are considered natural water purifiers.
Why It Matters for Climate and People
These cycles don’t just sit quietly in the background. They shape everything from the fertility of farmland to the stability of coastlines. If you’ve ever read about “dead zones” in the Gulf of Mexico, that’s a biogeochemical imbalance—too much nitrogen and phosphorus washing into rivers from fertilizers, fueling algae blooms that choke out fish.
On the bigger scale, carbon cycling between rocks, soils, oceans, and the atmosphere controls Earth’s climate. The U.S. Geological Survey and NOAA have decades of data showing how human activity—burning fossil fuels, deforestation, industrial farming—has accelerated these natural flows, often in destructive ways.
Human Disruptions
- Agriculture: Heavy fertilizer use throws nitrogen and phosphorus cycles out of whack.
- Mining: Rips open rock reservoirs, flooding soils and rivers with metals.
- Urbanization: Paves over soil, choking microbial activity and disrupting water infiltration.
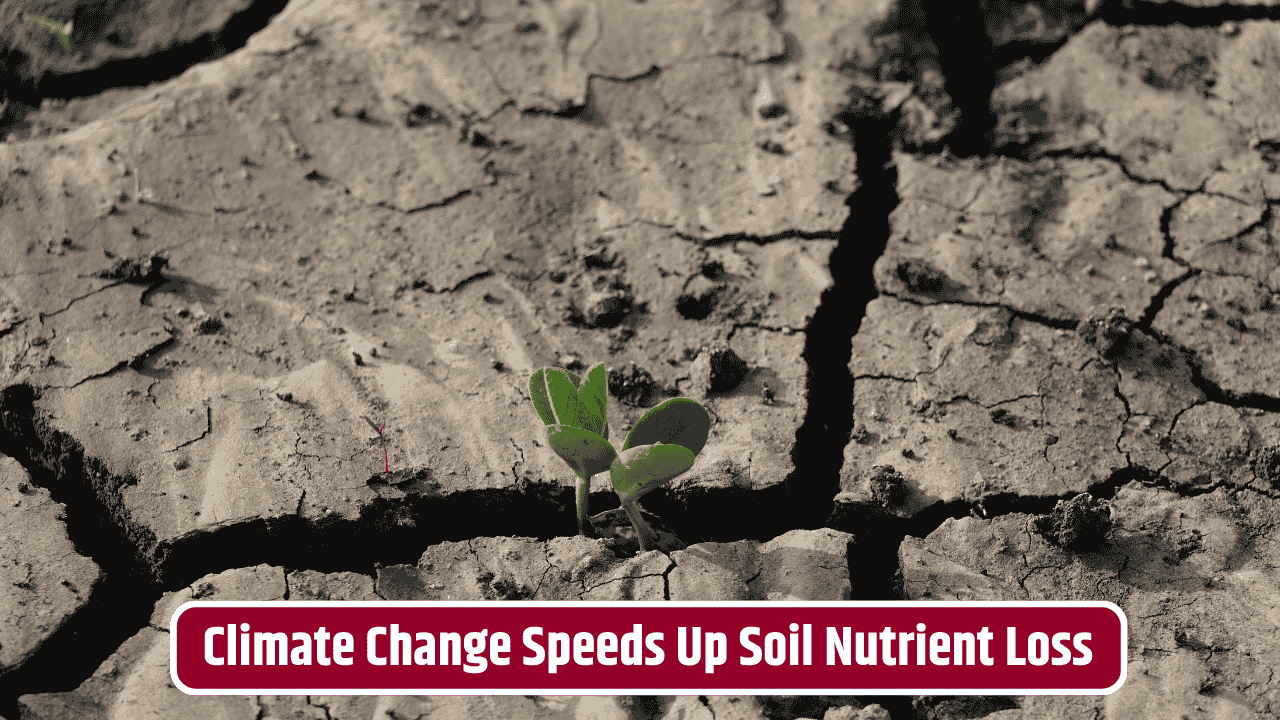
- Climate change: Alters rainfall and temperature patterns, speeding up or slowing down these cycles unpredictably.
Bringing It All Together
If you zoom out, Earth is one big chemistry set, and biogeochemical interactions are its stirring spoon. The balance between rock weathering, soil biology, and water chemistry is what lets forests grow, rivers stay fresh, and the air remain breathable. When humans meddle too much, those balances tip—and the consequences are not subtle. Think dust bowls, algal blooms, or poisoned groundwater.
So the next time you feel that earthy smell after rain, remember: it’s not just “nature being nature.” It’s a giant, ancient conversation between rock, soil, water, and life—and we’re very much a part of it.
FAQs
What’s the simplest example of a biogeochemical cycle?
The water cycle itself—evaporation, condensation, precipitation—though it’s often coupled with chemical and biological processes.
How long do these cycles take?
Some are lightning-fast (microbes breaking down leaf litter in days), while others like rock weathering can stretch over millions of years.
Are these interactions visible to the naked eye?
Sometimes—like rust-colored streams (iron oxidation) or algal blooms—but much of it happens microscopically.
Can we restore balance if humans disrupt a cycle?
Yes, through methods like wetland restoration, reduced fertilizer use, and sustainable land management, but it takes coordinated effort.
Which element cycle is most critical today?
Carbon and nitrogen cycles are under the most human pressure, with direct ties to climate change and water pollution.